Yunfeng Chen, PhD
Yunfeng Chen, PhD
Assistant Professor, Department of Biochemistry & Molecular Biology
Tel: (409) 772-0027
E-mail: yunfchen@utmb.edu
Mail Route: 0645
Research
"Mechano-medicine" is an emerging concept that tries to prevent and cure diseases by combining mechanobiology with biomedicine. The Chen lab focuses its research on the interface of mechanobiology, vascular biology and biomedicine. We use multidisciplinary approaches to 1) understand the mechanobiology of cells and molecules, including force-regulated molecular binding and conformational change, cell adhesion and mechano-signaling, in the vascular system; 2) shed light on the mechanobiology-related pathogenesis of vascular dysfunctions like thrombosis, atherosclerosis and cancer metastasis; and 3) eventually, develop mechanobiology-inspired therapeutics and research/diagnostic tools abiding the concept of ‘mechano-medicine’. The approaches of Chen lab can be summarized in seven M’s: Mechanics (investigating the mechanobiology of cell physiology and pathology); Microscopy (combining super-resolution imaging with force spectroscopy); Microfabrication (manufacturing microfluidic devices to realize high-throughput experimentation and develop mechanobiology-based diagnostic tools); Molecular engineering (designing and making protein mutants to understand disease pathology and explore potential therapeutic strategies); Molecular dynamics simulation (via collaboration, understanding the mechanisms underlying mechanobiological molecular behaviors); Mouse models (use transgenic mice to create disease models); and finally Mechano-medicine (designing and testing new therapeutics).
1. Receptor-mediated cell mechanosensing
We are investigating the mechanisms of different mechanosensing molecular machinery on the surface of varied vascular cells, primarily platelets but also including neutrophils, endothelial
cells and metastatic cancer cells. We aim to distinguish different proteins or protein domains in the mechanosensing system as modules to accomplish certain mechanosensing tasks and steps: mechano-presentation, mechano-reception, mechano-transmission
and mechano-transduction.
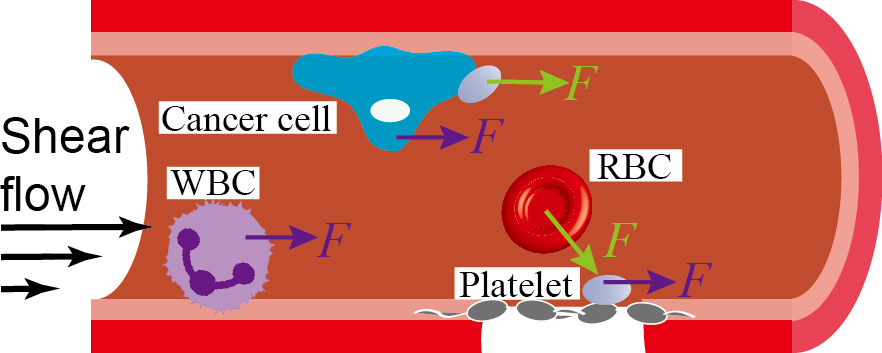
2. Vascular mechanomedicine
1) Arterial thrombosis, which describes the formation of abnormal blood clots in the artery, is a highly fatal disease that claims ~500,000 American lives per year. We are investigating how shear force-induced
platelet activation contributes to arterial thrombosis, and how to inhibit it to safely prevent arterial thrombosis from becoming lethal. Two platelet mechano-receptors, GPIbα and integrin αIIbβ3 have been identified to play critical
roles in platelet mechanosensing, and we are establishing novel strategies to suppress their activity.
2) Von Willebrand disease (VWD) is the most common inherited bleeding disorder. The pathogenesis of Type 2B and 2M VWDs are associated with
mutations in the VWF A1 domain (VWFA1) – the binding site for platelet receptor GPIbα. We are investigating how these mutations cause mechanobiological deficiencies in VWF in mediating hemostasis. Related to translational medicine, we
are bioengineering VWF to reinforce its hemostatic function, which will inspire a potent therapeutic approach for treating Type 2B and 2M VWDs.
3. Virus-related vascular dysfunctions
COVID-19 has been found to cause multiple dysfunctions in the vascular system, leading to thrombosis, thromboembolism and thrombocytopenia. However, the underlying mechanisms are not fully
understood. We are investigating how infection of SARS-COV2 affects the adhesion and mechano-activation of platelets and VWF production in endothelial cells. The mechanobiology of SARS-COV2 virus-host cell interaction is also studied to better understand
the viral infection process. Furthermore, we are exploring other viruses that cause vascular dysfunctions.
4. Integrin conformational changes and mechano-signaling
Integrins are mechano-receptors on cell surfaces that allow the cells to perform rigidity sensing and mechanosensing. We take the initiative to combine experimental and theoretical
approaches to establish a biophysical model that comprehensively describes how mechanical force regulates the binding and conformational changes of integrins, which can be used to predict how mutations, drugs and diseases will affect integrin mechanosensing.
5. High-throughput microfluidic platform development
We are developing novel microfluidics-based high-throughput platforms to evaluate cell adhesion and shear-induced thrombogenesis. The developed devices will be used for basic
science studies, disease diagnosis and drug screening.