Matthieu G. Gagnon, PhD
Associate Professor, Department of Microbiology & Immunology
Tel: (409) 772-2326
Fax: (409) 772-2366
E-mail: magagnon@utmb.edu
Campus Location: 4.172 Medical Research Bldg
Mail Route: 1019
Research
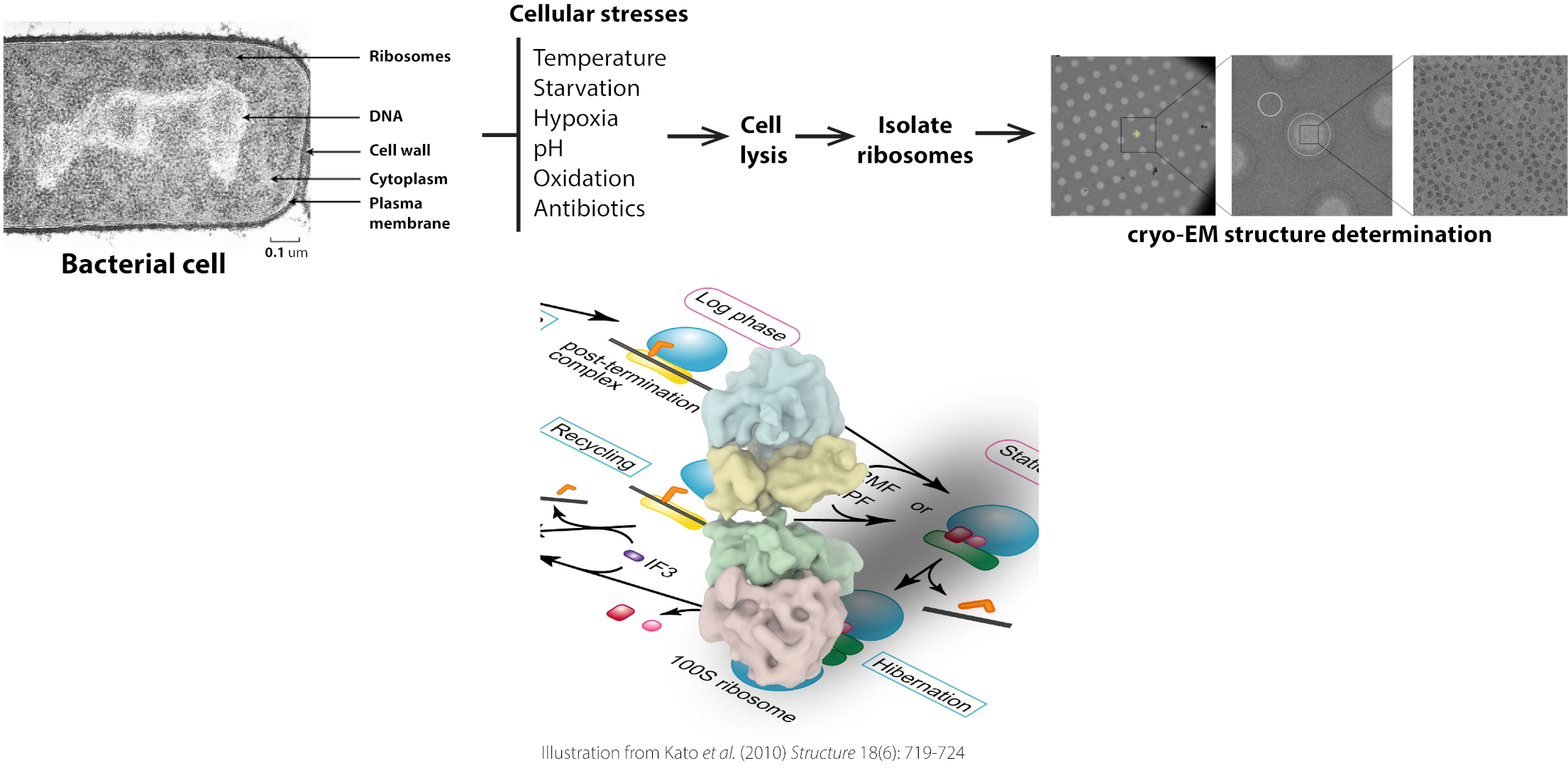
The Gagnon laboratory studies the mechanisms of protein biosynthesis and the basic cellular processes regulating translation by elucidating high-resolution structures of ribosome functional complexes. The overarching goals of our research are to elucidate the molecular mechanisms bacterial cells use to regulate protein synthesis during normal and stress growth conditions. Various stresses are being studied using the model bacterium Escherichia coli, and many other human pathogens, such as Mycobacterium tuberculosis and Pseudomonas aeruginosa. To achieve our goals, we use an integrated approach that combines biochemistry, microbiology, molecular biology, protein chemistry, genetic, transcriptomic, proteomic, and kinetic approaches with structure determination techniques such as cryo-electron microscopy (cryo-EM).
To learn more about our current projects, visit the Gagnon Lab.