Petr G. Leiman, PhD
Associate Professor, Department of Biochemistry & Molecular Biology
Tel: (409) 747-2078
E-mail: pgleiman@utmb.edu
Campus Location: 5.104B Basic Science Bldg
Mail Route: 0647
Pubmed Publications | Google Scholar | Lab Webpage
Research
Our ultimate research goal is to describe how large dynamic macromolecular complexes (biological nanomachines) work in quantitative terms. Understanding the structure of these machines in atomic detail is key to achieving this goal. We use a combination of X-ray crystallography and electron microscopy with other biochemical and biophysical techniques. The following systems are of interest from the basic, fundamental science side or because of their importance in technological or medicinal applications.
Bacterial type VI secretion system (T6SS)
Bacteria use secretion system to deliver complex substrates - large toxins and enzymes, which alter cellular functions - in the external milieu and sometimes directly into the cytoplasm or periplams of target host cells. Seven distinct types of secretion systems (type I through VII), have been characterized today. Compared to types I through V, relatively little is known about the function and structure of the type VI secretion system (T6SS). Despite being defined as a secretion system very recently, the T6SS cluster of genes is present in more than 25% of all known bacterial genomes making T6SS one of the most widespread secretion systems. T6SS appears to be a sole determinant of pathogenicity of many bacterial strains. T6SS proteins assemble into a large tubular structure spanning the cytoplasm and the envelope of the cell. Several T6SS proteins are structurally and functionally related to those found in contractile tails of bacteriophages. The tail is a complex organelle, responsible for host recognition, penetration through the multilayered host envelope, and delivery of phage DNA and proteins from the phage capsid into the cytoplasm. The accumulated data suggest that a similar protein complex is assembled by the T6SS proteins.
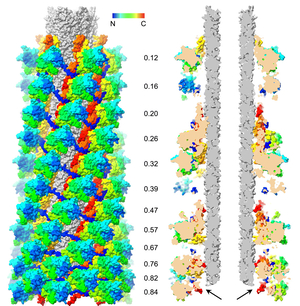
R-type pyocins
In addition to secretion systems, some bacteria use various bacteriocins to suppress growth of other cells occupying the same niche. Many strains of the widespread pathogen, Pseudomonas aeruginosa, can produce pyocins, very potent bacteriocins, which are formed by one or several protein molecules. The R-type pyocins are homologs of contractile phage tails and, in turn, are related to the T6SS machine. The pyocins represent a very potent proteinatious bactericidal, which can be retargeted to a new host. These features make the R-type pyocins a prospective lead as a therapeutical antimicrobial. Our aim is to understand how R-type pyocins function at the atomic level of detail.
Bacteriophages
We study the structure and functional mechanism of host adsorption organelles of certain complex bacteriophage.