Research
The research in the laboratory involves work in the areas of : (1) Biochemistry, (2) Chemical Physics/Physical Chemistry, and (3) Computational Science. We study:
 Bacteriophages, found in bacteria-rich locations like rivers and soil,
are nature's machinery for viral infection of bacteria. Their genetic
material, DNA or RNA, single- or double-stranded, are carried in
protein-based capsids and released into the bacteria. Understanding the
biophysical basis of the biological process which transfers a viral
genome to infect a cell is important to the cellular machinery and many
disease related fields. Predicting the thermodynamic pressures including
the osmotic pressure necessary to confine DNA in a specific volume,
like a phage, is a problem with implications in genomics,
nanotechnology, infection, phage therapies and therapeutic delivery.
DNA, a charged elastic polymer, undergoes over 250-fold compaction when
packed into a capsid overcoming an unfavorable thermodynamic barrier by
using ATP. How DNA overcomes the unfavorable thermodynamic barrier to
enter and pack inside a capsid depends on the interplay of many
different intermolecular interactions. Combined with experimental data,
coarse-grained models and multi-scale techniques are being employed to
model the structure and, consequently, the thermodynamics of DNA
confined by surfaces.
Bacteriophages, found in bacteria-rich locations like rivers and soil,
are nature's machinery for viral infection of bacteria. Their genetic
material, DNA or RNA, single- or double-stranded, are carried in
protein-based capsids and released into the bacteria. Understanding the
biophysical basis of the biological process which transfers a viral
genome to infect a cell is important to the cellular machinery and many
disease related fields. Predicting the thermodynamic pressures including
the osmotic pressure necessary to confine DNA in a specific volume,
like a phage, is a problem with implications in genomics,
nanotechnology, infection, phage therapies and therapeutic delivery.
DNA, a charged elastic polymer, undergoes over 250-fold compaction when
packed into a capsid overcoming an unfavorable thermodynamic barrier by
using ATP. How DNA overcomes the unfavorable thermodynamic barrier to
enter and pack inside a capsid depends on the interplay of many
different intermolecular interactions. Combined with experimental data,
coarse-grained models and multi-scale techniques are being employed to
model the structure and, consequently, the thermodynamics of DNA
confined by surfaces.
Phase transitions in protein solutions. How and why proteins fold is a problem that has implications for protein design and therapeutics. Several groups have had some success in describing some aspects of the problem, such as folding a sequence. However, the discovery that proteins do not always necessarily fold into a single stable structure calls for a redefinition of both the folding problem itself and the mechanisms we use to describe it. We consider commonly used concepts of protein folding in relation to solubility and phase transitions in solution. The formation of many non-enveloped cellular structures are governed by the underlying rules of solubility.
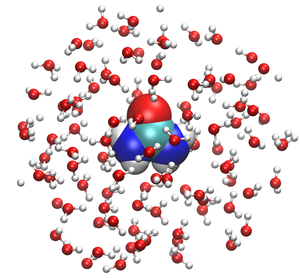 Thermodynamics
and kinetics in liquid solutions especially aqueous systems. Most difficult is the question of how multicomponent systems including crowding, cosolvents and ions affect proteins and nucleic acids in solution. Given correlations and statistical thermodynamics
the relations to experimental observables on the effects ions and osmolytes have on biomacromolecules in solution should then be understandable. At the technical level we are working on activity models and diagramatic expansion.
Thermodynamics
and kinetics in liquid solutions especially aqueous systems. Most difficult is the question of how multicomponent systems including crowding, cosolvents and ions affect proteins and nucleic acids in solution. Given correlations and statistical thermodynamics
the relations to experimental observables on the effects ions and osmolytes have on biomacromolecules in solution should then be understandable. At the technical level we are working on activity models and diagramatic expansion.
 Theory
and computational methods to investigate solution systems with couplings and correlations at many disparate length and time scales. There are many problems for which atomic correlations do not provide a direct link to macroscopic properties. Connecting
meso scale averaging procedures to the atomic and macro levels via multiscale methods is important for biological/materials applications.
Theory
and computational methods to investigate solution systems with couplings and correlations at many disparate length and time scales. There are many problems for which atomic correlations do not provide a direct link to macroscopic properties. Connecting
meso scale averaging procedures to the atomic and macro levels via multiscale methods is important for biological/materials applications.