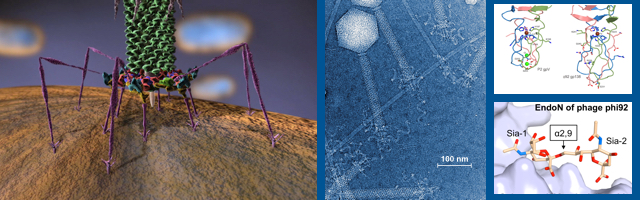
Welcome to the Leiman Lab
We use a combination of X-ray crystallography and electron microscopy with other biochemical and biophysical techniques. Individual proteins and their complexes are crystallized and analyzed using X-ray crystallography. The entire assembly is cryo-fixed and imaged with a high-end electron microscope. These images are then used to calculate a three-dimensional map of the entire assembly. The X-ray atomic structures of the components can be placed into the electron microscopy map, similar to a jigsaw puzzle, giving rise to a pseudo-atomic resolution structure of the entire assembly. Such an approach results in a tremendous amount of information and allows us to understand the structure and function in atomic detail.
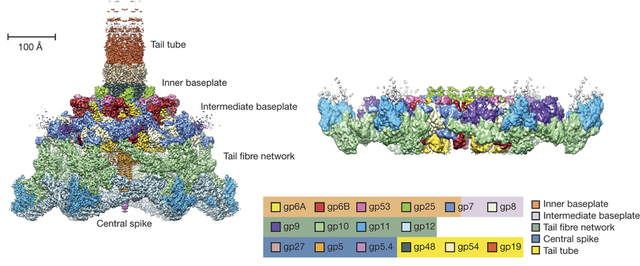
We study the structure and function of large dynamic macromolecular complexes (biological nanomachines) comprised of hundreds of protein molecules. Of particular interest are the bacterial type VI secretion system (T6SS), R-type pyocins, and the host cell adsorption organelles of bacterial viruses. These systems employ a rigid tube plus contractile sheath mechanism for breaching the envelope of the target host cell and are capable of translocating large proteins and DNA across lipid membranes.